Closure Devices

Features
- Easy to Use
- Suitable to various LAA anatomies
- Fully recapturable and repositionable within LAA
- Patented anchor design to ensure stable device fixation
- 8-10 Fr. delivery sheath
Stent Graft System
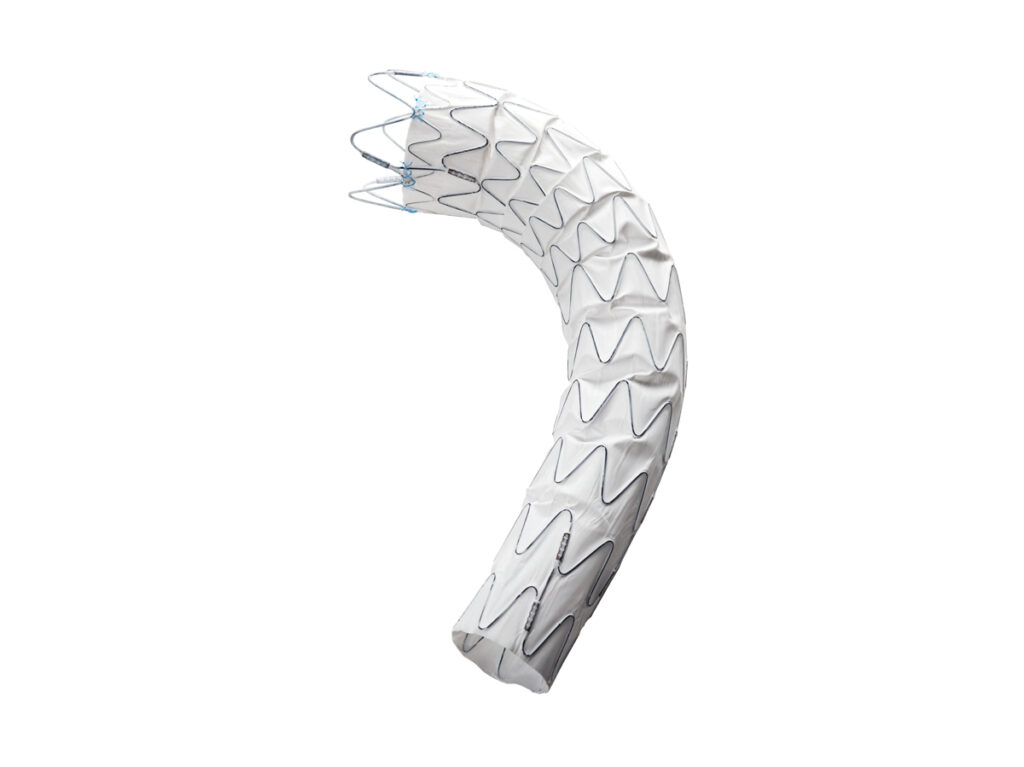
The Ankura™ Thoracic Stent Graft is specifically designed for endovascular treatment of thoracic aortic dissection, aneurysm and other relative diseases. It reinforces the vascular wall of thoracic aorta from rupture.
Optimal Proximal Bare Stent
•Allow for deployment across the LSA or LCCA without occluding the blood flow
•Provide extended Proximal Landing Zone
•Less traumatic to the aortic branches
Proximal Sealing and Fixation
•MINI Wave – Enhanced apposition to the aortic wall
•Decreased endoleak risk
•Migration resistant
Greater Flexibility
•Conform to the natural curvature of thoracic aorta
•Reduce the possibility of kinking
•4mm, 6mm and 8mm taper available
Other Strengths
•Self-expanding Nitinol stent – Radial force fixation
•No suture on the main body – Avoid pinhole leakage ( Type Ⅳ endoleak)
•Longitudinal supporting strut on the greater curvature – Avoid stent shortening, provide axial support
•e-PTFE Dual Membrane – Excellent biocompatibility and durability
•Kink-resistant delivery system with Hydrophilic Coating
Information intended for Patients
Magnetic Resonance Imaging (MRI)
A patient after being implanted with the Ankura Stent Graft can be safe to have MRI procedure under the following conditions:
– Static magnetic field of 3.0 Tesla or less
– Spatial gradient filed of ≤720Gauss/cm
– Maximum whole-body-averaged specific absorption rate (SAR) of 2W/kg for 15 minutes of scanning
Expected lifetime of Ankura Device
The Ankura Stent Graft is a permanently implanted device. Under normal circumstances, it will stay in the patient’s body for life, unless it is required to be removed by the doctor’s professional judgment. Lifetech evaluated the durability and integrity of the implanted device. After 10 years simulated physiological loading of 380 million cycles of the implanted device, tested samples were examined visually under 3D microscope with magnification (20x). There was no evidence of Nitinol wire pitting or cracking, nor of fatigue related fractures. The device was intact after 10 years simulated in vivo physiological loading of 380 million cycles, and no perforation or detachment of the ePTFE graft as a result of pulsatile fatigue test.
Follow up
It is important to schedule regular follow-up visits with your doctor. Follow-up visit will help the doctor to check your aneurysm/dissection and stent graft on a regular basis. The follow up visit should be performed at 24 hours, 1, 3, 6, 12 and 24 months after the procedure, and can be adjusted by the doctor depending on your condition.
At each visit, imaging such as CT scans will be carried out to determine the performance of the stent graft. If you have poor kidney function, you should ask your doctor about the dyes used in some of these imaging studies, as they may be harmful.
Material/Substances in Contact with Patient
The materials and substances to which the patient can be exposed in the implantable device (in wt.%) are NiTi (Nickel titanium, 52-67%), stainless steel (7-16%), PtIr (Platinum-Iridium, 0.5-6%), PTFE (Polytetrafluoroethylene, 20-40%), PP(Polypropylene,<1%).< p=””>
The Ankura Stent Graft implants are composed of Nitinol (48-58%w/w), Stainless steel (6-8%w /w), Polytetrafluoroethylene (PTFE, 35-43%w/w), Platinum-Iridium (<1%w/w) and Polypropylene (PP, <1%w/w). During the intended use, the thoracic aorta, abdominal aorta, iliac artery, femoral artery as well the blood will come in contact with the device.
Note: If you have a history of metal allergies, you should ask your doctor. Your doctor will help you decide whether it is appropriate for you to get an Ankura stent graft.
The Ankura devices do not contain medicinal substances, animal or human tissue; they are no blood products and are not radioactive.
Delivery System

Outstanding Design
Unique tip deflection technology
Combination of long sheath and guiding catheter
Outstanding tip deflection technology:0-160°
Precise Super Select Catheterization
Save surgery time by avoiding repetitive catheter sizing selection
Minimize vessel injury by avoiding repetitive catheterization procedures
onvenient for Usage
Quickly and easily access target using only one device
Short sheath + guiding catheter
Pre-shaped selective catheter + exchange operation + long sheath
Non-Invasive Position
Smooth transition between dilator and sheath
Avoid injury to vessel wall and vascular opening
Minimize risk of plaque obstruction during the advancement
Stable Support
Enhanced proximal segment
Ensure stable supporting for subsequent operations of device after access is established
Multiple Applications with only One Sheath
Specifications: 5F, 6F, 7F, 8F, 9F, 10F, 12F, 14F
Lengths Available: 550mm, 700mm, 800mm,900mm
Deflectable Lengths: 30mm, 50mm
Connector Type: Hemostasis valve
Suitable for a variety of interventional procedures, including structural heart disease, coronary artery disease, neurovascular, peripheral vascular disease and etc.
Delivery System

The Aegisy™ Vena Cava Filter is designed for percutaneous delivery into inferior vena cava (IVC) via the femoral or jugular approach under fluoroscopy or ultrasound guidance to offer protection from PE by capturing blood clots in DVT patients.
The Aegisy™ Vena Cava Filter can be permanently placed in the IVC for PE prevention or can be removed out of the patient after a period of time (up to and including 14 days) as a retrievable filter.
The Aegisy™ Vena Cava Filter is delivered with an Introducer Kit which contains a 6F Delivery Sheath, a Vessel Dilator, a 4.5F Delivery Cable and a 6F Loader. The Filter is connected with the Delivery Cable and preloaded in the Loader.
Precisely Controlled Release
There is one nut (with screw) respectively at the proximal and the distal end of the filter to allow attachment with the Delivery Cable.
Precise positioning, accurate deployment
Avoid “Jump forward” when released
Outstanding Filtration Capability
Asymmetric lantern design
– A three Y-shaped caudal entry basket
– A six diamond-shaped cranial basket-Filtration only at this layer
– With six struts connecting the two baskets
Large capacity for clot trapping
Transverse symmetry offers great stability of clot trapping
Desirable IVC Patency Rate
Asymmetric configuration-Filtration at only one layer
Minimize the effect to blood flow dynamics after clot capture
Central turbulent shear force enables the blood flow to break the clot apart
Prevent thrombus from spreading in VCF
Maintain desirable long-term IVC patency
Dual Access – Jugular or Femoral
The filter is preloaded for femoral use. Reassemble the device according to IFU before usage in jugular. The length of Introducer sheath is 55cm.
Easy to Retrieve (As Retrievable Filter)
High success rate of retrieval
Retrieving Hook – At the end of caudal basket
Using Snare system for retrieval
Retrieval time – Up to and including 14 days
Retrieve via the femoral vein only
Safe and Reliable
Laser Cut – Fatigue resistant
Highly Biocompatible
6F Delivery System reduces vessel injury
Six securing anchors -Fix filter to vessel wall
Six straight struts-Prevent device from tilting
Low incidence rate of migration or fracture
Vascular Plug

Important information:
Magnetic Resonance (MR) conditional
▶ Non-clinical testing showed that a patient with an implanted plug can be scanned safely immediately after placement of the device under the following conditions:
● Static magnetic field of 3 T or less.
● Spatial gradient magnetic field of 720s G/cm or less.
● Maximum MR system-reported, whole-body-averaged specific absorption rate (SAR) of 2 W/kg for 15 minutes of scanning.
▶ Based on non-clinical testing, the device was determined to produce a temperature rise of less than 1.5 °C at a maximum whole-body-averaged specific absorption rate (SAR) of 2.21 W/kg for 15 minutes of MR scanning. The maximum whole-body-averaged specific absorption rate (SAR) was derived by calculation and verified by calorimetry.
▶ MR image quality may be compromised if the area of interest is in the same area or relatively close to the position of the device. Therefore, optimization of MR imaging parameters to compensate for the presence of this device is necessary. The image artifact extends approximately 15 mm outside the device lumen when scanned in non-clinical testing using the spin echo sequence and gradient echo sequence, respectively in a 3.0 T Scanner with a whole body coil.
Material/Substances in Contact with Patient
The materials and substances to which the patient can be exposed in the implantable device (concentration in wt.%) are: NiTi (Nickel titanium, 54%-68%), stainless (9%-37%), TiN (Titanium Nitride, ~0%), PA (Polyamide, 0%-1%), PTFE (Polytetrafluoroethylene, 8%-23%).
The expected lifetime of the device, including data on implant survival rates:
The device is a permanently implanted device. A fatigue testing to simulate in vivo use of the device for 10 years was performed and the results are satisfied: the metal wire of vascular plug has no breaks or cracks, and the connection points of the metal wires are not loose, the plug is not migrated and its head is intact. Under normal circumstances, it will stay in the patient’s body for life, unless it is required to be removed by the physician’s professional judgment. No special attention is needed by the patient.
Follow-up
It is important to schedule regular follow-up visits with your doctor. Follow-up visit will help the doctor to check your heart on a regular basis. The follow up visit should be performed at 24 hours, 1, 3, 6, and 12 months after the procedure, and can be adjusted by the doctor depending on your condition.
Sizing Ballon
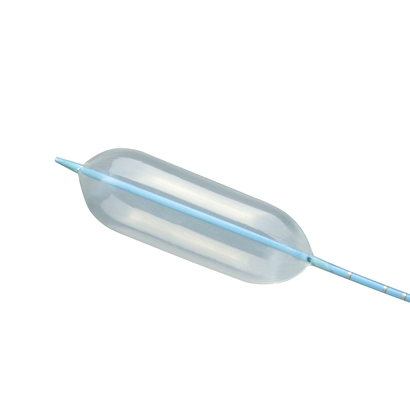
The AcuMark™ Sizing Balloon (Lifetech) is a double-lumen catheter which is designed to measure cardiovascular defects such as an ASD.
Positioning message
·Soft compliance material
·Easy to use
·Precise measurement
Four radiopaque markbands at 10 mm, 5 mm and 2mm intervals (as measured from leading edge to leading edge) are used as a distance reference for magnification correction.
·Option for different size- 3 codes cover all kind of defect size
-Up to 18mm
-Up t0 28mm
Dilator

The LawMaxTM Dilator is intended to dilate the puncture tunnel of the skin, subcutaneous tissue and vessel.
One Step Dilatation-no need to change dilators
Handle design-user friendly
Hydrophilic coating-reduces coefficient of friction and facilitates dilator introduction
Radiopaque visualization-easy to identify the depth of the dilation
Snare System

Titanium Nitride Coated Tungsten Loop
-Increased biocompatibility
-Enhanced fracture resistance
-Excellent radiopacity
-Excellent shape supporting and distal shaft control ability
Flexible Nitinol shaft provides Kink Resistance and 1:1 Torque control
90°angle between Loop and Shaft makes manipulation and retrieval more simple
Radiopaque marker at distal tip of catheter provides excellent Fluoroscopic visualization
A wide range of usage for clinical procedures
